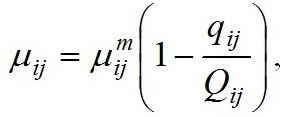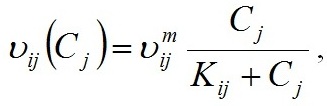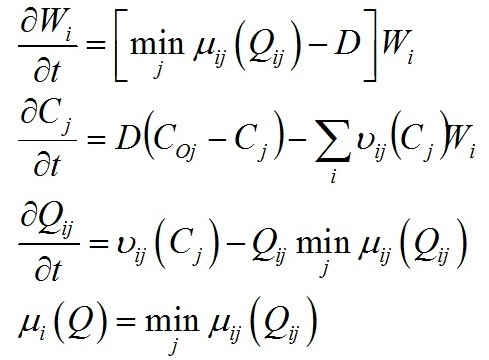|
|
|
Свойства ключевых видов фитопланктона Черного моря, определяющие смену их доминирования в годовой динамике
Чехутский С.Г., Лифанчук А.В. Sergey G. Chekhutskiy, Anna V. Lifanchuk
Институт океанологии им. П.П. Ширшова РАН, Южное отделение (Геленджик, Россия) Shirshov Institute of Oceanology RAS, Southern Branch (Gelendzhik, Russia)
УДК 581.1:574.522
С помощью математической модели проведена проверка трех гипотез механизмов регулирования «цветения» доминирующих видов фитопланктона в северо-восточной части Черного моря. В основу модели положена концепция внутриклеточного регулирования роста с использованием уравнения Друпа и найдены значения констант модели, удовлетворяющие условиям доминирования. Показано, что в ранний весенний период «цветение» мелкоклеточной диатомеи Pseudo-nitzschia pseudodelicatissima обусловлено более высокими, чем у других видов, удельными скоростями роста. Константы полунасыщения кинетики потребления биогенных элементов (азота и фосфора) и минимальные клеточные квоты для этих элементов также относительно высоки. В поздний весенний период и в начале лета обычно регистрируется «цветение» кокколитофориды Emiliania huxleyi. Необходимым условием являются низкие значения константы полунасыщения для поглощения азота и минимальная квота для этого элемента. Третий механизм набирает силу летом при заглублении сезонного термоклина и доминировании крупноклеточных диатомей. Показано, что для доминирования диатомеи Proboscia alata необходимо иметь низкие константы полунасыщения для поглощения фосфора и минимальные квоты по этому элементу. Ключевые слова: математическое моделирование; диатомовые водоросли; кокколитофориды; элементы минерального питания; Pseudo-nitzschia pseudodelicatissima; Proboscia alata; Emiliania huxleyi.
Using mathematical model, we tested three hypotheses of bloom regulation mechanisms for dominant phytoplankton species in the northeastern Black Sea. The model is based on the concept of intracellular regulation using the Droop equation, and values of the model constants that satisfy the conditions of domination are found. The bloom of the small-cell diatom Pseudo-nitzschia pseudodelicatissima is due to higher specific growth rates than other species in early spring. The half-saturation constants and minimum quotas for mineral nutrition elements (nitrogen and phosphorus) are also relatively high. In late spring and early summer, the bloom of coccolithophore Emiliania huxleyi is usually recorded. Prerequisites are a low half-saturation constant for nitrogen uptake and minimum quota for this nutrient. The third mechanism gains strength in the summer with the deepening of seasonal thermocline and the dominance of large-cell diatoms. It was shown that for the supremacy of large diatom Proboscia alata, it is necessary to have low half-saturation constants for the phosphorus uptake and minimum quotas for this nutrient. Keywords: mathematical modeling; diatoms, coccolithophores; nutrients; Pseudo-nitzschia pseudodelicatissima; Proboscia alata; Emiliania huxleyi .
Introduction Biomass and abundance of phytoplankton dominants, as well as the community structure, are subject to regular seasonal changes. These changes in the structure of the community are due to seasonal variations in the water dynamics, temperature, irradiance, and nutrients concentrations. Phenotypic characteristics of dominant phytoplankton species are the next factor that influences community dynamics because of the ability of the species to respond to external changes may be crucial (Litchman et al., 2015). Changes in the complex of dominant species are critical both for analysis and prediction of changes in the marine ecosystem, as well as for the identification of succession mechanisms. The material on seasonal dynamics of phytoplankton in the northeastern part of the Black sea was used to identify the mechanisms of succession and to study the influence of cell physiology in combination with external factors. Diatoms and coccolithophores are the basis of the phytoplankton community in the northeastern part of the Black sea (Pautova et al., 2007). The contribution of dinoflagellates and species of other taxonomic groups usually is not significant. Having examined the mechanisms in “diatoms-coccolithophores” system, we can understand the mechanisms of functioning of the entire community. Coccolithophore mainly represented by Emiliania huxleyi. This species dominates in late spring and early summer (Pautova et al., 2007; Silkin et al., 2009; Mikaelyan et al., 2011). The spring bloom is typical of annual phytoplankton successions marine ecosystem (Barton et al., 2013; Sommer et al., 2012; Winder and Cloern, 2010). In early spring, the small-cell diatom Pseudo-nitzschia pseudodelicatissima dominates. The large-cell diatoms Pseudosolenia calcar-avis and Proboscia alata dominate in summer and autumn (Silkin et al., 2013; Silkin et al., 2019b). Based on long-term field observations, it was shown that small-cell diatoms dominate at relatively high nitrogen and phosphorus concentrations, coccolithophores at relatively low levels of nitrogen, large-cell diatoms at relatively low levels of phosphorus (Silkin et al., 2019a). Experiments have shown that the main factors of phytoplankton growth in the Black sea are the concentrations of nitrogen and phosphorus, and the nitrogen to phosphorus ratio largely determines structural shifts (Silkin et al., 2013, 2014; Lifanchuk, 2017). Mathematical models are a useful tool for predicting the responses of marine phytoplankton to changes in nutrient inputs and other environmental factors (Sarthou et al., 2005; Shoener et al., 2019; Sunda et al., 2009). At the moment, mechanisms of regulation of the “diatoms – coccolithophores” system are not fully understood. In particular, it is not clear what physiological properties the species must have to ensure dominance. These traits are reflected in model constants. Two models based on different concepts are used to describe phytoplankton growth (Silkin et al., 2016; Sunda et al., 2009). The first concept of extracellular regulation matches specific growth rate and nutrients concentration in medium and is described by the Monod equation (Flynn, 2010; Silkin, Khaylov, 1988). The second concept of intracellular regulation divides growth into two processes: nutrients uptake and biomass accumulation, i.e., biosynthesis. The biosynthesis process is determined by the intracellular content of growth-limiting nutrient. The Droop equation describes this process (Droop, 1968, 1974). In the current study we use mathematical model which is based on the concept of intracellular regulation. The main goal of this work was to determine the constants of the model that ensure the dominance of three species. In other words, we tried to answer the question of what properties should small-cell diatom Pseudo-nitzschia pseudodelicatissima, coccolithophore Emiliania huxleyi, and large-cell diatom Proboscia alata possess to win a competition from other species under appropriate environmental conditions.
Materials and methods Mathematical modeling Dynamics of the “diatoms – coccolithophores” system in the upper mixed water layer (UML) isolated from the rest water column by a seasonal thermocline are simulated. It is considered depending on nitrogen and phosphorus concentration in the water at the upper boundary of seasonal thermocline. The process of nutrients assimilation is divided into two – nutrient uptake and growth of biomass. Specific growth rate is determined based on the Droop model (1968; 1974). This model assumes that the growth rate is a function of intracellular content of the nutrient: μij and μijm are the specific and maximum specific growth rates of the i species on the j nutrient; Qij and qij are the current and minimum content of the j nutrient in the biomass of the i species. When cells grow, Q > q always and the maximum growth rate can not be exceeded. The Michaelis-Menten model determines specific uptake rate of the nutrient (Silkin, Khaylov, 1988): where υij and υijm are the specific and maximum specific nutrient uptake rates of the j by species i; Cj designate amount of the nutrient j; Kij is the half-saturation constant for nutrient j in species i. The system of equations describes the dynamics of species and nutrients concentrations in the UML at a different exchange rate D through seasonal thermocline: Functions Wi and Cj designate concentrations of species biomass i and the amount of nutrient j, respectively; СOj – concentration of nutrient j at the border of thermocline; D – the rate of exchange of the water in the UML. Together with equations (1) and (2), the system takes the full form. In this model, the behavior of the “diatoms – coccolithophores” system is modeled for three physiological mechanisms of bloom mentioned above. The solution to the operation of differential equations was based on the modified Rosenbrock formula of order 2 (Rosenbrock, 1963). For this work, we used parameters specific to species in the northeastern part of the Black Sea (table 1). The initial conditions for species biomass were assumed to be equal, which is generally not true. Still, for this work, this question is not decisive and can be omitted. The effect of the initial conditions will be investigated in coming papers. The maximum specific growth rate for each species was taken based on estimates of this parameter obtained when cultivating natural assemblages of the Black Sea phytoplankton during the seasons of this dominance. Other parameters were evaluated using the selection method.
Results From the results of numerous computational experiments, estimates of parameters that meet the conditions of species dominance are found (Silkin et al., 2019b) (table 1). Parameters i and j were replaced by names of species and nutrients accordingly (3.1–3.4). Table 1. Parameters used for the simulation of phytoplankton community
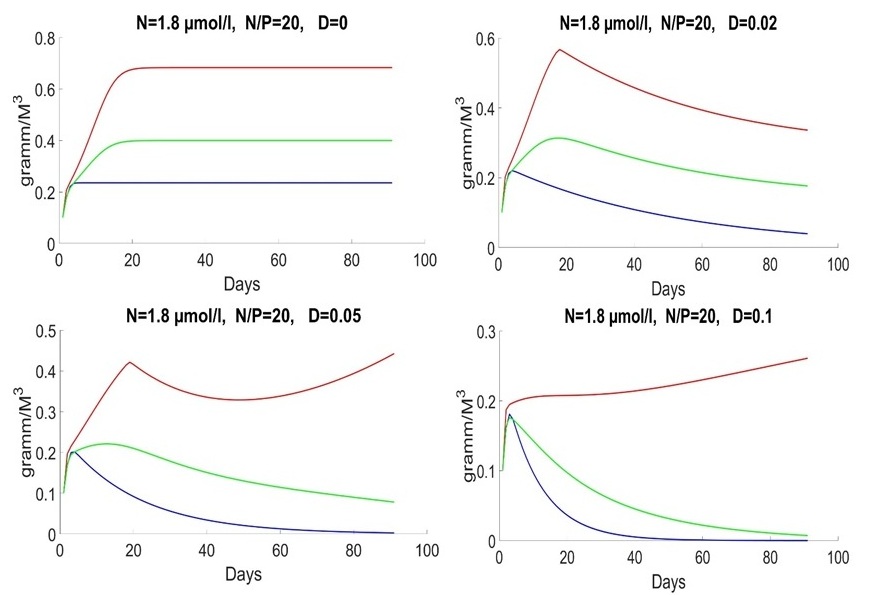
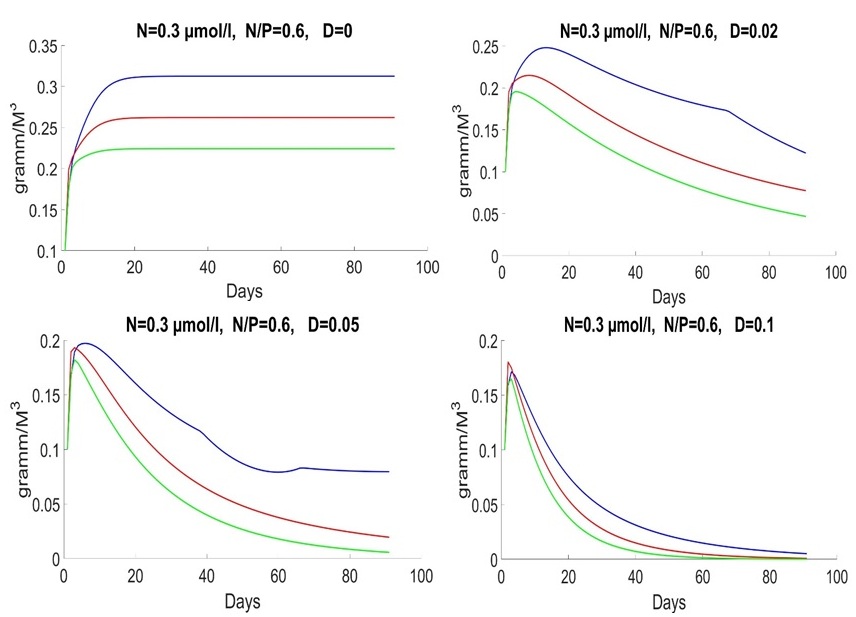
An initial biomass of each species of 0.1 g/m3 was used. The initial values of the mineral nutrition elements varied in each experiment. The early spring period is characterized by an average nitrogen concentration of about 1.8 mmol/l and a nitrogen-to-phosphorus ratio of about 16 (Silkin et al., 2019b). Under these conditions, the competition is won by Pseudo-nitzschia pseudodelicatissima at exchange rates from 0 to 10% (Fig. 1), which corresponds to field research. This species must have high growth rates compared to other species to provide these competitive advantages, which is confirmed by experimental studies. The half-saturation constant for nitrogen absorption is higher than that of Emiliania huxleyi but less than that of Proboscia alata: The minimum nitrogen quota also follows this ratio: As for the half-saturation constant for phosphorus absorption, the reverse trend is observed in comparison with nitrogen – the maximum value of this parameter in coccolithophores: A minimum quota for phosphorus also follows this trend:
Fig. 1. Dynamics of Pseudo-nitzschia pseudodelicatissima (red line), Emiliania huxleyi (blue line) and Proboscia alata (green line) in the spring period at different rates of medium exchange (day-1)
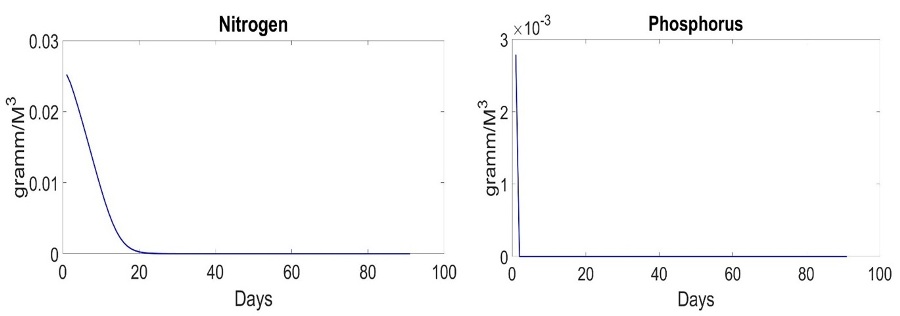
Fig. 1 shows behavior of the system in the early spring period with different parameters of D. D=0 represents ideal case. It does not exist in the real-world but provides us with a possibility to analyze process of uptake on a standalone basis. D=0 means that system does not get nutrients outside. The biomass of species does not decrease by result of water flow. The system grows till moment of end of nutrient and stabilizes in the end. The amount of nutrients reduces, as shown in Fig. 2.
Fig. 2. Dynamics of nutrients in the system with D=0
Finally, the model does not imply mortality. That is why species stabilized with D = 0. We will consider parameter D in the next articles. In other words, the theory assumes a stationary state. Specific speed is equal to zero due to uptake, and nutrients are equal to zero as well. Late spring and early summer are characterized by a low nitrogen concentration of 0.3 mmol/l, and the ratio of nitrogen to phosphorus is significantly lower than 16. Under these conditions, Emiliania huxleyi wins the competition (Fig. 3).
Fig. 3. Dynamics of Pseudo-nitzschia pseudodelicatissima (red line), Emiliania huxleyi (blue line) and Proboscia alata (green line) in late spring and early summer at different medium exchange rates (day-1)
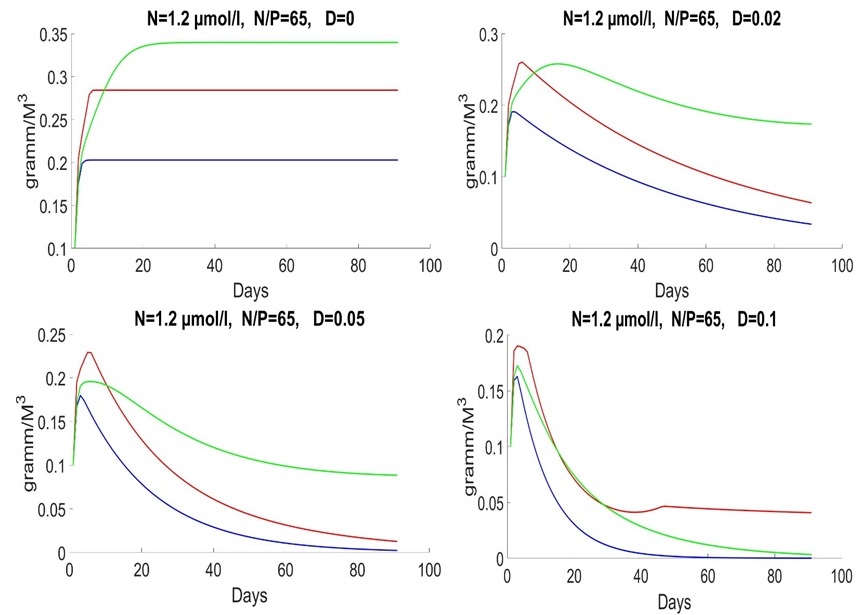
For the late summer period, the average nitrogen content of 1.2 mmol/l is characterized by very high values of the nitrogen-to-phosphorus ratio. The value 50 was used for illustration. During this period, Proboscia alata develops most intensively (Fig. 4).
Fig. 4. Dynamics of Pseudo-nitzschia pseudodelicatissima (red line), Emiliania huxleyi (blue line) and Proboscia alata (green line) in summer and autumn at different rates of medium exchange (day-1)
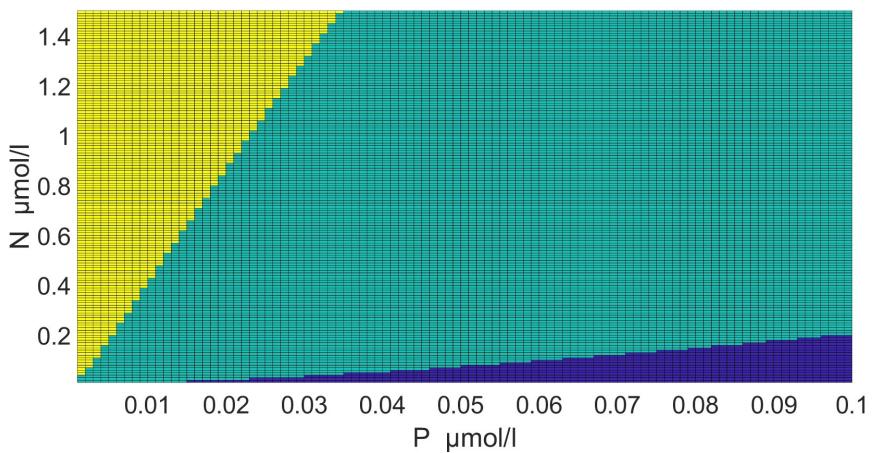
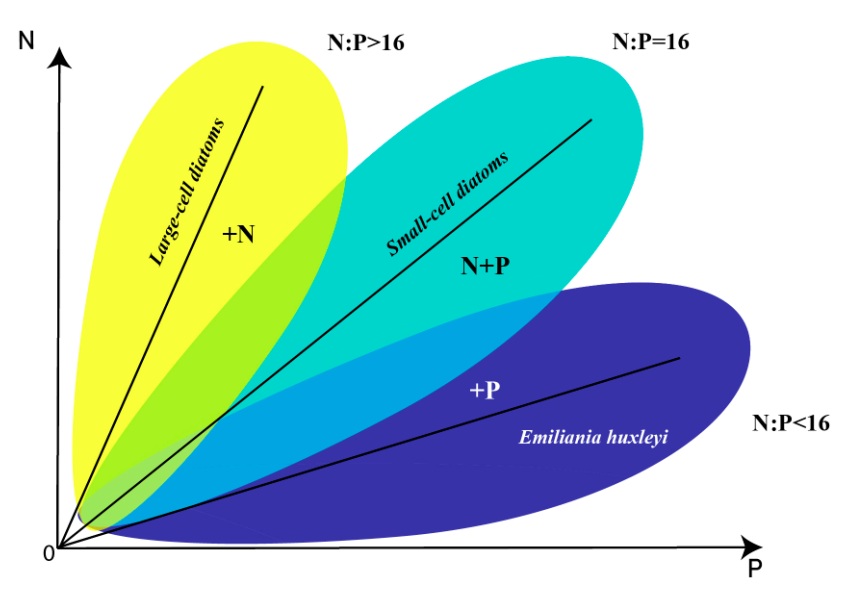
The areas of the dominance of individual species in the coordinates of nitrogen and phosphorus concentrations were determined using this model with parameters (table 2) (Fig. 5).
Fig. 5. Areas of the dominance of Pseudo-nitzschia pseudodelicatissima (turquoise), Emiliania huxleyi (blue) and Proboscia alata (yellow) in the coordinates of nitrogen and phosphorus concentrations in the medium
Discussion The data collected in the Russian part of the Black Sea show that the succession in the community “diatoms – coccolithophores” is designed so, that since the spring is dominated by diatoms (Pseudo-nitzschia pseudodelicatissima, etc.), then coccolithophore (Emiliania huxleyi) may begin to bloom, and since the end of June large-cells diatoms (Proboscia alata) lead. This process of succession is most typical for the specified area but is not invariant, i.e., there are years when there are significant deviations from it (Silkin et al., 2019b). For example, in some years, E. huxleyi dominated in winter or bloom began in April (Sukhanova, 1995; Kubryakov et al., 2019; Mityagina, Lavrova, 2019). But in most cases, it is the above order that was observed. Although the factors that influence the structure of this mechanism are not fully understood, there is a reason to believe that the determining factors are a group of factors consisting of internal (the physiological parameters of the phytoplankton species themselves) and external environmental factors (wind speed and direction, temperature, light intensity, as well as the ratio of nitrogen to phosphorus and their absolute values). Our research has shown that two external factors, namely the concentration of nitrogen and phosphorus, are sufficient to describe the dynamics of phytoplankton adequately. All others are auxiliary. Indeed, for low horizontal advection, the vertical transport of food elements is crucial (Barton et al., 2014). This transfer mechanism depends on mixing intensity, i.e., wind intensity. Given that the maximum concentrations of nitrogen and phosphorus are at different depths, wind acts as an essential factor in regulating the structure of phytoplankton (Silkin et al., 2019b). A relatively simple model of phytoplankton dynamics was used. Still, it describes the behavior of the “diatoms – coccolithophores” system well. It corresponds to data collected in the Russian segment of the Black Sea (near the city of Gelendzhik). Increase in wind speed observed in winter (Moskalenko et al., 2011) leads to increased mixing intensity and hence increase in nutrients concentrations in the UML and, consequently, to more intensive growth of small-cell forms, since high specific growth rates and acquisitions characterize these species, and half-saturation constant and a minimum quota for nitrogen and phosphorus. This mechanism is also confirmed by mathematical models (Fig. 1). It is the early spring period that is characterized by the absolute dominance of the small-cell diatom Pseudo-nitzschia pseudodelicatissima. The decrease in wind intensity in late spring and early summer leads to a reduction in the nutrients concentrations in the UML. And there is a change of dominant species in the community structure. The coccolithophorid Emiliania huxleyi replaces the small-cell diatom Pseudo-nitzschia pseudodelicatissima. According to our assumption, the dominance of this species is due to its physiological properties, which allows it to function successfully at low nitrogen concentrations. As a result, coccolithophorid E. huxleyi wins the competition from diatoms P. pseudodelicatissima and Proboscia alata. Mathematical characteristics are expressed in low values of growth rate, absorption rate, half-saturation constant and minimum nitrogen quota. Usually, starting from the end of June, mixing increases, which leads to the sinking of the thermocline to nitrogen-rich layers (Silkin et al., 2019b), and Proboscia alata begins to dominate. Physiological mechanisms that lead to this dominance have not been fully elucidated. Still, field studies suggest that this species dominates when nitrogen content is high compared to phosphorus. Mathematical modeling confirms that P. alata dominates at N/P>25. Presumably, this phenomenon is due to the high capacity of cells to accumulate nitrogen and then to use them for growth processes. In this case, it is necessary to divide the process of metabolism into the process of uptake of growth-limiting nutrients and growth itself. Species that win competition under these conditions must have a low half-saturation constant for phosphorus uptake and a minimum quota for this element. As can be seen from the graphs, the model describes well the behavior of the “diatoms – coccolithophores” system. It corresponds to the conceptual scheme of diatoms and coccolithophores dominating areas of the northeastern part of the Black Sea (Fig. 6).
Fig. 6. A conceptual diagram of phytoplankton dominance regions obtained from field and experimental studies according to Silkin et al., 2019a
Thus, high concentrations of nitrogen and phosphorus lead to the dominance of small-cell diatoms, in particular, Pseudo-nitzschia pseudodelicatissima. When nitrogen content is low, coccolithophores (Emiliania huxleyi) predominate. The increased nitrogen content compared to phosphorus leads to the leadership of the large-cell diatom Proboscia alata. These species dominate in the phytoplankton community in the northeastern part of the Black Sea due to the corresponding parameters, namely minimum quota, half-saturation constant and maximum uptake rate.
The author claims that there is no conflict of interest that requires disclosure in this article. This work was carried out within the framework of the state assignment of Ministery of High Education and Science of Russia Federation (theme № 0149-2019-0014).
Список литературы
Статья поступила в редакцию 09.02.2020
Об авторах Чехутский Сергей Геннадьевич − Chekhutskiy Sergey G. инженер, Институт океанологии им.П.П.Ширшова РАН, Южное отделение, Геленджик, Россия (Shirshov Institute of Oceanology RAS, Southern Branch, Russia, Gelendzhik) sergcheh@gmail.com Лифанчук Анна Викторовна − Lifanchuk Anna V. кандидат биологических наук
Корреспондентский адрес: Россия, 353470, Краснодарский край, г. Геленджик, ул. Просторная 1-г. Телефон/факс 8-861-41-280-89.
ССЫЛКА НА СТАТЬЮ: Чехутский С.Г., Лифанчук А.В. Свойства ключевых видов фитопланктона Черного моря, определяющие смену их доминирования в годовой динамике // Вопросы современной альгологии. 2020. № 1 (22). С. 82–93. URL: http://algology.ru/1583 DOI - https://doi.org/10.33624/2311-0147-2020-1(22)-82-93 Уважаемые коллеги! Если Вы хотите получить версию статьи в формате PDF, пожалуйста, напишите в редакцию, и мы ее вам с удовольствием пришлем бесплатно. При перепечатке ссылка на сайт обязательна
Properties of main phytoplankton species of the Black Sea, determining the change Sergey G. Chekhutskiy, Anna V. Lifanchuk Shirshov Institute of Oceanology RAS, Southern Branch (Gelendzhik, Russia) Using mathematical model, we tested three hypotheses of bloom regulation mechanisms for dominant phytoplankton species in the northeastern Black Sea. The model is based on the concept of intracellular regulation using the Droop equation, and values of the model constants that satisfy the conditions of domination are found. The bloom of the small-cell diatom Pseudo-nitzschia pseudodelicatissima is due to higher specific growth rates than other species in early spring. The half-saturation constants and minimum quotas for mineral nutrition elements (nitrogen and phosphorus) are also relatively high. In late spring and early summer, the bloom of coccolithophore Emiliania huxleyi is usually recorded. Prerequisites are a low half-saturation constant for nitrogen uptake and minimum quota for this nutrient. The third mechanism gains strength in the summer with the deepening of seasonal thermocline and the dominance of large-cell diatoms. It was shown that for the supremacy of large diatom Proboscia alata, it is necessary to have low half-saturation constants for the phosphorus uptake and minimum quotas for this nutrient. Keywords: mathematical modeling; diatoms, coccolithophores; nutrients; Pseudo-nitzschia pseudodelicatissima; Proboscia alata; Emiliania huxleyi.
References
Authors Chekhutskiy Sergey G. ORCID - https://orcid.org/0000-0002-4212-8825 Shirshov Institute of Oceanology RAS, Southern Branch, Russia, Gelendzhik sergcheh@gmail.com Lifanchuk Anna V. ORCID - https://orcid.org/0000-0001-9953-7374 Shirshov Institute of Oceanology RAS, Southern Branch, Russia, Gelendzhik
ARTICLE LINK: Chekhutskiy S.G., Lifanchuk A.V. Properties of main phytoplankton species of the Black Sea, determining the change in their dominance in annual dynamics. Voprosy sovremennoi algologii (Issues of modern algology). 2020. № 1 (22). P. 82–93. URL: http://algology.ru/1583 DOI - https://doi.org/10.33624/2311-0147-2020-1(22)-82-93 Dear colleagues! If you want to receive the version of the article in PDF format, write to the editor,please and we send it to you with pleasure for free. When reprinting a link to the site is required
К разделу ОБЗОРЫ, СТАТЬИ И КРАТКИЕ СООБЩЕНИЯ

|
|||
|
| ||